HATU (1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate, Hexafluorophosphate Azabenzotriazole Tetramethyl Uronium) is a reagent used in peptide coupling chemistry to generate an active ester from a carboxylic acid. HATU is used along with Hünig's base (N,N-diisopropylethylamine, DIPEA), or triethylamine to form amide bonds. Typically DMF is used as solvent, although other polar aprotic solvents can also be used.

HATU is commonly encountered in amine acylation reactions (i.e., amide formation). Such reactions are typically performed in two distinct reaction steps: (1) reaction of a carboxylic acid with HATU to form the OAt-active ester; then (2) addition of the nucleophile (amine) to the active ester solution to afford the acylated product.
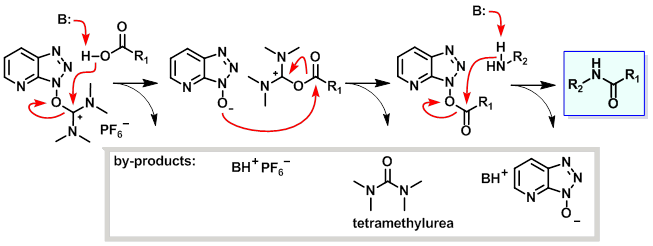
The reaction mechanism of carboxylic acid activation by HATU and subsequent N-acylation is summarised in the figure below. The mechanism is shown using the more commonly encountered and commercially available iminium isomer; a similar mechanism, however, is likely to apply to the uronium form. In the first step, the carboxylate anion (formed by deprotonation by an organic base ) attacks HATU to form the unstable O-acyl(tetramethyl)isouronium salt. The OAt anion rapidly attacks the isouronium salt, affording the OAt-active ester and liberating a stoichiometric quantity of tetramethylurea. Addition of a nucleophile, such as an amine, to the OAt-active ester results in acylation.
The high coupling efficiencies and fast reaction rates associated with HATU coupling are thought to arise from a neighbouring group effect brought about by the pyridine nitrogen atom, which stabilises the incoming amine through a hydrogen-bonded 7-membered cyclic transition state.